Plating is considered one of the most used surface finishing processes in multiple industries. Metal plating is used to make the components corrosion resistant, improve solderability, increase hardness, reduce friction and improve paint adhesion. Plating not only offers longevity to the components but also gives it a sleek and smooth finish. It is used on trim, fasteners, and mechanical components for durability and appearance.
Contents
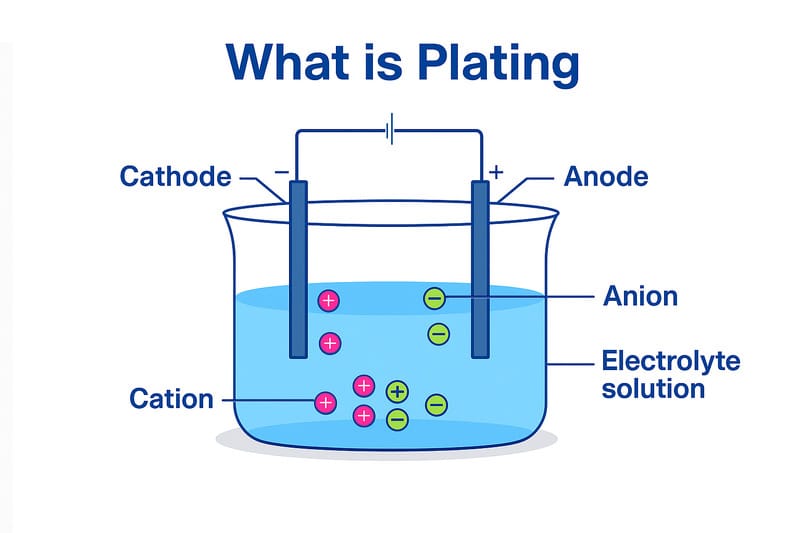
What is plating?
Plating usually refers to coating a thin layer of metal over the other material surface that can be metal or non-metal. The thickness of coated metal ranges from 1–50 μm for electroplating. For this purpose, the components to be plated are dipped into an electrolyte solution containing the ions of the metal to be coated. Usually, these solutions are aqueous, but molten salt can be used sometimes.
The components here act as cathode, and an electrode of metal is used. As the reaction begins either by applying current, such as electroplating, or without external current, such as in electroless plating, the metal ions in the solution begin to deposit on the surface of the components. This results in a smooth surface with enhanced corrosion resistance. The deposition of ions can be done in two different ways according to the technique used:
- Metal ions become physically trapped in microscopic pores on a surface, such as mechanical interlocking.
- At the boundary between materials, van der Waals forces help molecules adhere or spread out across surfaces.
What is the Plating Process?
Here is a step-by-step plating process:
Preparing the Surface
The first step in plating is to ensure that the surface of components is clean. The surface of the component is cleaned by a degreasing and abrasion process to make it free from any dirt debris or corrosion.
Activating the Component’s Surface
Now, the surface of components is treated with acid or some chemical solution to ensure its reactivity. This step ensures whether the surface will react with desired metal ions or not.
Preparing the Plating Solution
It is considered one of the most important steps in plating as it includes preparation of a solution which contains the metal ions to be coated on the surface. The choice of solution components depends on which type of plating is required, either chrome plating, rhodium plating, or gold plating.
Dipping into the Solution
Now the components are dipped into the solution and further procedure continues accordingly depending on whether it is electroplating or electroless plating. In electroplating, the external current is applied, whereas, in electroless plating, an autocatalytic reaction occurs for the deposition of metal on the surface.
Post Treatment
After the plating process is complete, the components are finally taken out of the solution and rinsed with water to remove any unnecessary plating chemicals from the surface. The components are dried and subjected to post treatment such as polishing to give it a shiny final look.
Plating Methods
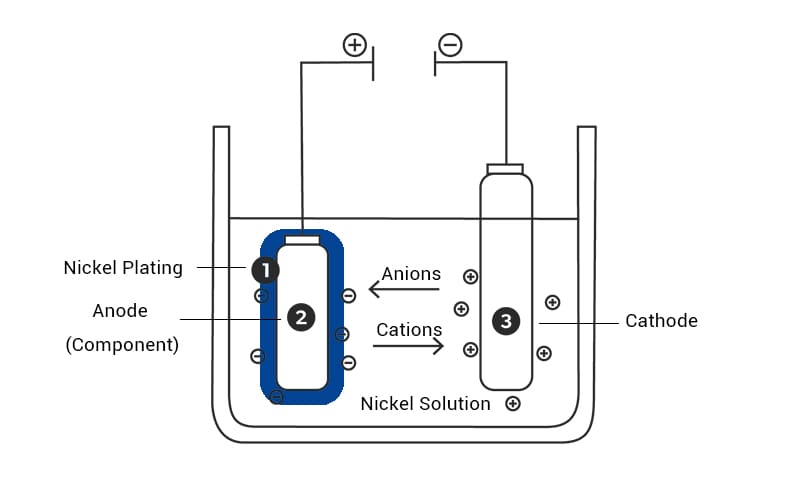
ElectroPlating
In electroplating, a direct current (DC) power source is used to deposit a layer of metal over a conductive surface. Here, the substrate acts as the cathode, attracting metal ions from the solution. The metal ions are reduced and deposited as a thin metallic film. In electroplating the
Electroplated metals usually include nickel, chromium, copper, gold, and silver. Electroplating deposition is used where precise control of thickness and material composition is required, such as in aerospace, electronics, and medical devices.
Types of Electroplating
Depending upon the required surface coating, the electroplating has been divided into various types, such as:
- Gold Plating
It is known for its exceptional conductivity and corrosion resistance. Gold plating found its application in electronics, aerospace, and medical devices. The thickness of gold plating varies depending upon the application such as in aerospace, its thickness is >2.5 µm.
- Silver Plating
Silver plating is performed where high electrical and thermal conductivity of the surface is required. It is considered ideal for RF connectors and switches. Sometimes, silver plating may require anti-tarnish treatments due to sulfide formation in the air.
- Nickel Plating
Nickel plating is known for its excellent wear resistance, hardness, and corrosion protection. Electroless nickel plating is applied for uniform coating or Watts nickel for a shiny, ductile finish.
- Chromium Plating
Traditional chrome baths rely on hexavalent-chromium electrolytes. These baths deposit a bright, wear-resistant metallic chromium layer.Because Cr⁶⁺ compounds used in traditional chrome plating are highly toxic and carcinogenic, the industry is shifting toward safer alternatives. Trivalent-chromium (Cr³⁺) systems reduce health risks while maintaining the gloss, hardness, and durability expected from chrome plating.
- Zinc and Zinc-Alloy Plating
When you need cost-effective sacrificial corrosion protection for steel, there comes zinc plating. It offers eight times better corrosion resistance and provides both finishing and resistance to corrosion.
- Copper Plating
Copper plating is quite economical and versatile; it finds its application in electronics due to its superior electrical and thermal conductivity. Copper plating is also used as an undercoat for materials such as nickel or chrome.
- Rhodium Plating
Rhodium plating finds its application in jewelry and high end electronics because it provides both a bright finish and enhanced wear resistance.
- Tin Plating
Tin is used extensively in electronics and food-safe applications for its non-toxic nature. Tin plating can be done in bright or matte finishes.
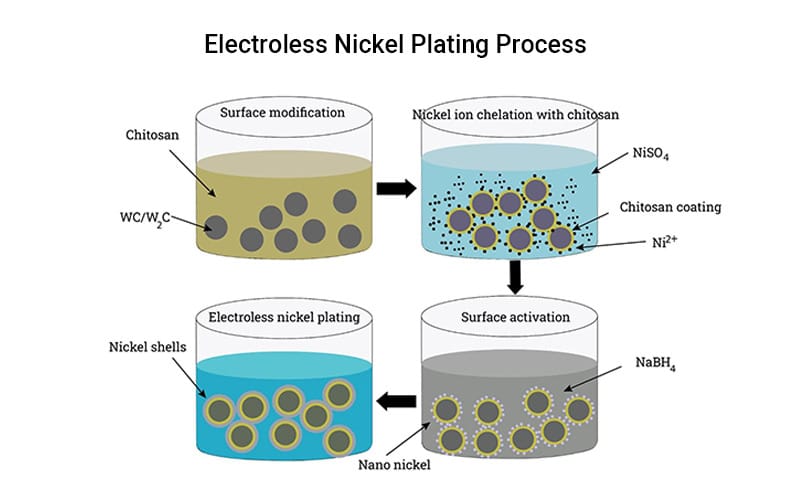
Electroless Plating
Electroless plating, also called autocatalytic plating, deposits metal through a self-sustaining redox reaction, so no external electrical current is required.
Typical thickness of electroless plating ranges from 1 to 120 μm. It is more costly and sensitive to process control than electroplating. It is preferred when consistent coverage and specialized surface properties are required, such as in aerospace.
Immersion Plating
Immersion plating includes deposition of a thin layer of metal over other substances through displacement reaction. Immersion plating offers a simple surface finishing process, but it is limited to some specific materials, such as gold or silver. Immersion plating is preferred when thin, even coatings are required on small or intricate parts.
Anodizing
Anodizing is an electrochemical oxidation process that enhances the surface of metals. It is mostly used for coating a layer of Aluminum by forming a durable oxide layer. Anodizing finds its application in various industrial sectors such as aerospace, architecture, and automotive.
Thermal Spraying
Thermal spraying plating begins by heating a material to be coated and propelling it onto a substrate to form a thick, protective barrier. It’s used in high-wear environments like turbines, pipelines, and engines. But the process can be expensive and produce rough surfaces that may require finishing.
Hot Dipping
In hot dipping, the metal components are immersed in a molten metal bath for a thick coating. The process is cost-effective and protective, especially for outdoor exposure. But Hot dipping can lead to uneven thicknesses of the coated surface.
Physical Vapor Deposition (PVD)
In PVD, the material is vaporized in a vacuum chamber and then deposited in the form of a thin layer on the substrate. It’s commonly used in automotive, tool, and decorative applications for finishes requiring hardness, wear resistance, and visual appeal.
Chemical Vapor Deposition (CVD)
In CVD, the solid coating is deposited by thermally decomposing vaporized chemicals in a reaction chamber. The coatings done by using this method are highly durable and uniform. One limitation of CVD is that it requires high temperatures and costly setups, which may limit substrate compatibility.
Plasma Spray Coating
Plasma spraying, as the name indicates, uses an ultra-hot plasma torch to melt materials. This molten material is then sprayed onto a surface to form a thick protective layer. This plating technique is considered ideal for aerospace, automotive, and biomedical applications, especially where heat and wear resistance are critical.
What Type of Material Can be Plated
Plating can be applied to a wide range of materials, but the effectiveness of the process depends on:
- The material’s conductivity
- Surface properties
- Compatibility with the plating method.
Here are the most commonly plated material types:
Metals (Conductive Materials)
These are the most common substrates for plating these include:
- Steel and Iron are usually plated with zinc .
- Aluminum plated with nickel or chrome
- Copper
- Brass and Bronze
- Titanium and Stainless Steel
Non-Metals
Although non-conductive materials cannot be electroplated directly, they can still be plated after surface activation. These materials include:
- Plastics
- Ceramics and Glass
- Composites
- Graphite and Carbon-Based Materials

Standards for Plating
Here are the commonly used standards for plating and their application:
| Standard | Focus | Typical Use |
|---|---|---|
| ASTM B633 | Zinc electroplating for corrosion protection | Automotive, machinery, hardware |
| ISO 4527 | Nickel and electroless nickel coating requirements | Electronics, aerospace, tools |
| MIL-STD-1500 | Plating quality control for military parts | Aerospace, defense systems |
| RoHS | Restricts hazardous substances in coatings | Electronics, consumer products |
| NACE MR0175 / ISO 15156 | Corrosion resistance in sour environments | Oil & gas, petrochemical components |
What is Plating Used For?
Improve Corrosion Resistance
Plating acts as a protective barrier that shields the base metal from exposure to moisture, oxygen, chemicals, and other corrosive elements. In this way, it protects the component’s surface from corrosion buildup.
Decorate Objects
Plating is widely used for decorative purposes to enhance the appearance of metal objects. Silver and chrome plating is used in automotive parts to give them an attractive, shiny finish.
Wear Resistance
It also enhances the wear resistance of the components’ surface, reducing the frequency of repairs and replacements.
Increase Solderability
Some surfaces are difficult to solder because of their chemistry. Such surfaces are subjected to plating, enhancing their solderability.
Reduced Friction
Plating provides a smoother, more rigid surface that reduces friction between moving parts. Chrome plating is usually done on bearings or gears to reduce wear due to friction.
Altered Conductivity
Plating is also useful for altering the conduction properties of components’ surfaces. Usually, silver and gold plating provide excellent electrical conductivity and corrosion resistance.
Enhanced Paint Adhesion
The surface is often plated with a layer such as nickel or zinc to enhance the adhesion of paints and coatings. These metals provide a clean, uniform surface with strong bonding properties that help the paint adhere more effectively.

Advantages of Plating
- Plating offers excellent corrosion protection, such as zinc-nickel plating, which can withstand up to 720 hours of salt spray testing.
- Provide excellent wear resistance, such as hard chrome reaching up to 70 HRC.
- It also enhances electrical conductivity of the surface. The electrical conductivity of the surface is enhanced by reducing contact resistance and RF losses in high-frequency applications.
- Provides extended component life, especially in harsh environments.
Limitations of Plating
- Some coating, such as high-strength steel, can lead to hydrogen embrittlement, which can cause cracking under load.
- Plating can lead to uneven coating in complex geometries due to current density variations.
- Some electroplating processes, such as hexavalent chromium, use toxic chemicals that are harmful to both human health and the environment.
- Plating can suffer from adhesion issues when poor surface preparation or incompatible base materials.



