According to the Industry Arc reports, the global tin plating market is predicted to hit around USD8.9 billion by the year 2030. Keeping the compound annual growth rate (CAGR) at about 4.2% from 2023 to 2030. This growth can be said to result from the continuous demand in industries like electronics, automotive, telecommunications, and even food packaging.
Tin is soft, with a combination of silver and white. It is known for it malleability and ability to resist corrosion. Due to its non-toxic nature, it is a top choice for use in the food industry for packaging purposes. Tin is highly conductive and very solderable. These properties make it preferable for projects that require high precision like production of circuit board and connectors.

Contents
What Is Tin Plating?
Tin plating, also called tinning, is a surface coating process where a thin layer of tin (Sn) is applied to a metal part, typically through electroplating or hot-dipping. The base metals commonly used include steel, copper, or iron. A product known as tinplate is the end result. This is especially useful in improving both the corrosion resistance and solderability of the base metal.
Benefits of Tin Plating
Tin plating provides the substrate with good corrosion resistance, excellent electrical conductivity, solderability, and ductility.
Corrosion Resistance
Tin plating provides a shield which protects against moisture, preventing rust and corrosion. Its non-reactive and non-toxic properties help prolong shelf life in different environmental conditions.
Conductivity
Its high conductivity makes it a top choice of use in coating electronic components, connectors, and terminals.
Excellent Solderability
You are sure to have an easier and more reliable soldering experience with a tin-plated surface. Its high solderability is of utmost importance for reliable bonding in electronics and circuit assemblies.
Cost‑Effective
Tin is way more affordable than precious metals like gold or platinum. If you’re looking to reduce production costs without compromising on quality, tin will make a smart choice.
Ductility
Tin is malleable. So, you can shape your tin-plated parts without worrying about cracks or breakage.
Tin Plating Process
The tin plating process entails the deposition of a tin coating onto the surface of a properly prepared metal substrate via the following steps:
1. Cleaning and Degreasing
First, thoroughly clean the metal you want plated. Using things like acid washing or abrasive cleaning. This ensures there is no dirt on the surface to help the tin bond better.
2. Pre‑Treatment to Aid Adhesion
After cleaning, you activate the metal with an acid solution to ensure an easier tin-bond. This step ensures the surface is ready to accept the tin layer.
3. Tin Plating Solution
A tin plating bath is prepared using either an acid or alkaline base solution. Key parameters to be monitored and adjusted accordingly include pH, temperature, and tin concentration.
4. Tin Plating Methods
Now, you immerse the metal in the bath using these methods:
Electro Tin Plating
Also called tin electroplating. You place the metal in the bath while passing electric current through it. This causes the ions to move towards the metal, hence, the bonding.
Sulfate tin plating is the most widely used because of the following reasons:
- Simple bath composition and process
- Low operating temperature (10-30°C)
- High current efficiency
- Energy-saving and low cost
- Easy control and maintenance
- Straightforward wastewater treatment
The following table shows the typical composition of stannous sulfate acid bath.
| Component | Matte Tin Plating | Bright Tin Plating |
|---|---|---|
| SnSO4 (g/L) | 40 – 100 | 40 – 100 |
| H2SO4 (g/L) | 30 – 80 | 70 – 170 |
| Gelatin (g/L) | 1 – 3 | / |
| β-Naphthol (g/L) | 0.5 – 1.5 | / |
| Brightener (mL/L) | / | 15 – 40 |
- Electroless Tin Plating
No external current needed, it is a chemical process which ensures uniform coverage. When you immerse the metal in the solution, a chemical reaction deposits the ions on the surface of the metal.
The main advantages of chemical tin plating are uniformity and high quality, while the disadvantages are low deposition rate and high cost.
- Hot‑Dip Tinning
Hot-dip tinning is the process of immersing a pretreated workpiece into a molten tin bath. This method enhances the corrosion resistance, wear resistance, and electrical conductivity of metals like copper and steel.
- Dipping temperature range: 260oC – 280oC
- Dipping time range: 6 – 10 seconds.
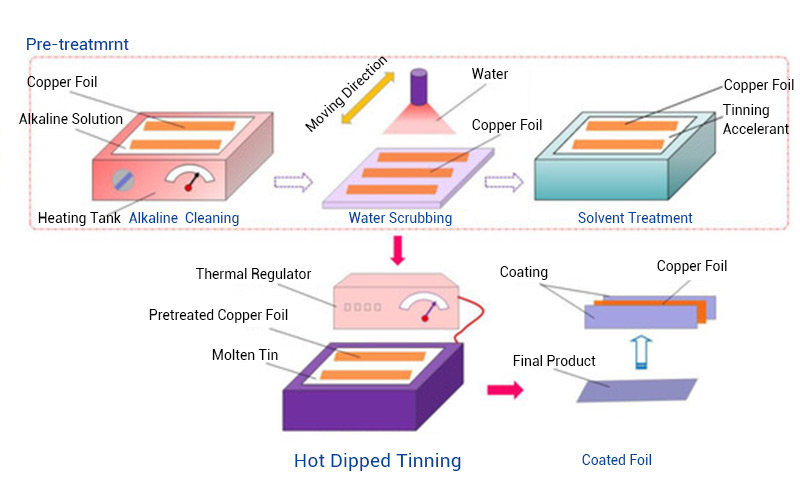
5. Post‑Plating Treatment.
After you finish plating, rinse the workpieces with running tap water or dilute alkali solution, then dry. Apply a coating of protective material (passivation) or some heat treatment for added corrosion protection. In addition, this also helps to prevent the workpieces from being weakened when exposed to hydrogen.
Types of Tin Plating
There are primarily two types of tin plating:
Bright Tin Plating
This type of plating has a fine grain structure (below 1 µm) with a finish that is mirror-like and shiny. It is used primarily for decorative purposes and electrical components such as terminals and busbars. However, it contains brighteners which cause burning under heat, hence, not suitable for soldering functions.

Matte Tin Plating
It is also known as dull tin plating. Contrary to bright tin plating, it has a coarse-grained, non-reflective surface. It is highly ductile and ideal for solderable applications, as it contains no brighteners and has whisker growth resistance.
Materials Suitable for Tin Plating
Tin plating can be done on both ferrous and non-ferrous metals.
- Steel & Stainless Steel: They protect against rust and are used for fasteners and busbars.
- Copper & Brass: Promote solderability on electronics.
- Aluminum: Requires special activation and is used for architectural components.
Tin Alloy Plating
Tin Alloy can simple be described as a combination of Tin and other metals. The variants help you explore a range of applications. Examples include, but are not limited to:
- Tin‑Lead Plating: Ensures ductility, and is widely used for soldering in electronics.
- Tin‑Zinc Plating: Great conductivity, provides a lubricating film, and is resistant to certain environmental factors.
- Tin-Nickel Plating: Ensures better corrosion and wear resistance than pure tin.
- Tin‑Gold Plating: After repeated use, you can depend on this combination for its reliability.
- Tin‑Silver Plating: A substitute for Tin-Lead due to its mechanical strength and improved resistance to thermal stress.
Tin Plating Uses
The following are common applications of tin plating.
Electronics
In this industry, tin plating is commonly used for coating terminals, printed circuit boards (PCBs), connectors, and other shielding components. Thanks to its good solderability and conductivity, electrical connections and assembling in high-precision electronic devices is made easy.
Automotive Industry
In this sector, tin plating enhances the durability and performance of components like busbars, battery terminals, connectors, and wiring systems. It improves corrosion resistance and electrical contact reliability, especially in electric cars.
Power and Renewable Energy
Tin plating is used for switchgears, transformers, solar panels, and wind turbines. This is because it helps extend the longevity of components by resisting corrosion and maintaining conductivity. Most especially, it’s known for maintaining stable electrical performance in outdoor and high-load environments.
Telecommunications
Tin-plated connectors and waveguides are critical in the telecommunications industry. Here, it is very crucial to have signal integrity and long-term reliability. The plating protects sensitive components from environmental wear and mitigates signal degradation.
Aerospace and Aviation
Aerospace systems need materials that can endure extreme environmental factors. For this reason, tin plating is used to make fasteners, connectors, and busbars due to its corrosion resistance. In addition, it ensures the integrity of critical systems throughout the life cycle of an aircraft.
Food Packaging
Tin’s non-toxic and corrosion-resistant properties make it useful in the food industry, specifically in the manufacturing of food cans. Tin plating helps preserve food by preventing contamination from the base metal and maintaining product safety.

Disadvantages of Tin Plating
Formation of Tin Whiskers
Tin whiskers are conductive tiny hair-like structures that can cause short circuits in electronic systems. You will most likely see them form on bright tin plating; however, the problem can be managed by introducing dull tin plating.
Low Temperature Operational Limit
You should know that tin plating has a low melting point, therefore, it is unsuitable for use at high temperatures. The operational limit of tin plating is usually around 200oC.
Short-lived Solderability
Tin plating can lose its solderability with time, if the metal substrate is not properly prepared during the plating process.
Possible Corrosion
You must be wondering, why corrosion? If the tinplate has scratches, it could lead to corrosion (especially on steel), due to the electrochemical potential difference between tin and steel.
Nickel vs Tin Plating
Tin plating offers advantages such as excellent electrical conductivity, good solderability, high ductility, and lower cost compared to nickel plating.
On the other hand, nickel plating provides higher hardness and a significantly higher melting point. Electrolytic nickel typically has an average hardness of around 300 HV and a melting point of 1455°C.
FAQ
1. Is Tin Plating Conductive?
Yes, tin is a highly conductive metal, making tin plating perfect for electrical applications.
2. What’s the Difference Between Electro Tin Plating and Electroless Tin Plating?
Electro tin plating uses electric current for faster, thicker deposition. Electroless plating uses chemical reactions with no electricity needed for even coatings and a simpler process.
3. What is ASTM B545?
ASTM B545 is a standard specification for electrodeposited coatings of Tin on metals, for solderability, corrosion-resistance and other properties. It only covers the requirement needed for Electrolytic tin plating and not hot-dip plating.
4. What is Barrel Plating?
A type of electroplating used for plating small components in large volumes with the use of a barrel. The barrel slowly rotates while being submerged in the plating solution.






