Copper plating uses an electrochemical or chemical process to deposit a thin layer of copper onto the surface of a prepared substrate, usually metal, but can also be plastic.
Copper plating is generally not used directly for decoration or protective outer layer, but as a base layer for nickel plating, chromium plating, gold plating, silver plating Since carbon and hydrogen are difficult to diffuse and penetrate in copper, the copper plating layer is also an excellent plating layer to prevent carburization and nitriding.

Contents
Copper Plating Process
Copper Electroplating
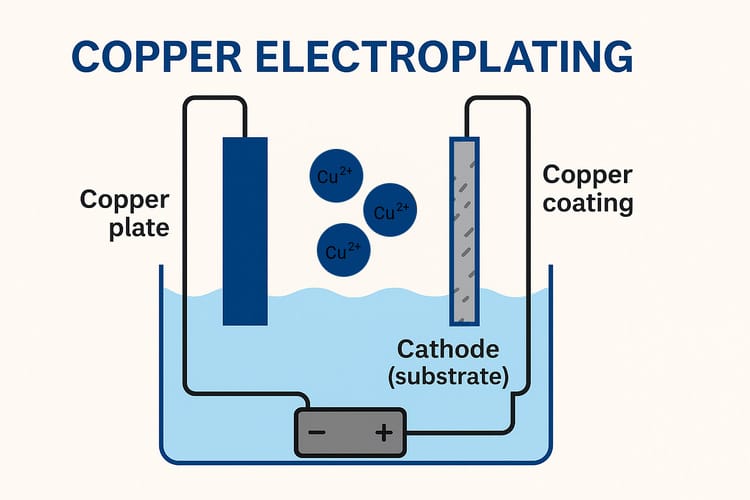
Copper electroplating is a process in which a thin layer of copper is deposited onto a substrate through an electrochemical reaction. In this method, the substrate to be plated acts as the cathode, while the copper plate serves as the anode.
Both the substrate and copper plate are immersed in the electroplating solution. During the electrochemical reaction, copper ions (Cu²⁺) are released from the copper anode into the solution, and a copper coating is formed on the cathode substrate.
The copper electroplating process consists of 4 key steps:
1. Cleaning
The process begins with rigorous cleaning to ensure a contaminant-free surface. Chemical cleaning agents, such as alkaline washes, are used to remove oils and dust.
If cleaning is not thorough, an oil film may remain on the surface, preventing proper bonding between the coating and the substrate. This may result in defects such as peeling and blistering.
2. Activation
After cleaning, the substrate undergoes activation, typically using an acid dip or micro-etching solution.
Activation removes the oxide layer and rust from the surface, fully exposing the base metal’s lattice structure and putting it into an activated state suitable for plating.
3. Electroplating
The cathode (workpiece) and copper anode are immersed in the electrolyte. A direct current is applied, and the bath temperature is maintained for optimal deposition.
Copper ions migrate from the anode through the solution and are reduced onto the cathode, forming a uniform metallic copper layer.
The thickness and quality of the deposit depend on current density, bath composition, and plating time. Typical deposit thickness ranges from 5 to 25 µm per cycle.
4. Finishing
After plating, the copper layer is polished and treated to enhance both appearance and durability.
Mechanical polishing or buffing is used to achieve the desired surface finish. Since copper is prone to oxidation, an anti-tarnish coating or passivation layer is applied to preserve its luster and prevent discoloration.
Electroless Copper Plating
Electroless copper plating is a chemical process that deposits a uniform copper layer onto both conductive and non-conductive substrates without the use of electrical current, relying instead on an autocatalytic reaction between copper salts and a reducing agent such as formaldehyde.
Electroless copper plating can uniformly cover the surface of the substrate. It is not affected by the size, shape and conductivity of the substrate, and is a very effective method for metallizing non-metallic surfaces. However, electroless plating is slower and more expensive than electroplating.
Types of Copper Electroplating
Acidic Copper Electroplating
Sulfate acid copper plating is mainly used for decorative electroplating, conductive plating, welding plating and as the final plating. Acid copper plating has good ductility and does not corrode certain plastics and printed copper foil adhesives like cyanide copper plating, so it is widely used in plastic electroplating and printed circuit board electroplating.
Acid sulfate copper plating has the following advantages:
- The plating solution composition is simple and the properties are stable
- The current utilization efficiency is close to 100%, and the deposition effect is good;
- Strong uniform plating and deep plating capabilities
- It can obtain copper plating with high ductility, high flatness and high brightness;
- The process is easy to control and the process operation is simple
- And it has less pollution to the environment.
Cyanide Copper Plating
Cyanide copper plating is a well-established electroplating process known for its excellent all-around plating capabilities, deep plating, good coating density, and ease of polishing. However, the cyanide system is highly toxic, difficult to decompose, and poses significant risks to both the environment and human health.

Pyrophosphate Copper Plating
The advantages of the pyrophosphate copper electroplating system are:
- Plating solution is stable
- It is easy to use in automated production
- Fine crystals
- Good surface gloss
- Good toughness
- No hydrogen embrittlement
- It can be directly electroplated on the steel surface
However, it also has the following problems:
- The bonding strength between the coating and the steel substrate is very poor
- The metal anode is easily passivated in the pyrophosphate electrolyte.
What is Copper Plating Used For?
Electroplating Copper Applications
Electronics
In the electronics field, a layer of copper is plated on the surface of the through-hole of the printed circuit board through the electroplating process to achieve the function of electrical conduction, which is conducive to the welding of small microelectronic devices.
Copper plating can also be used as the base plating of multi-layer metal plating of some metal parts or non-metal parts, such as the bottom plating of silver plating, gold plating, and tin plating. On the one hand, this can save precious metals. On the other hand, copper plating as an intermediate layer can enhance the bonding strength between the entire multi-layer plating and the base material.
Decoration
In addition, copper plating can be used as the surface of some decorations because of its smooth and delicate surface and good corrosion resistance.
Electroless copper plating Applications
Electroless copper plating is mainly used for printed circuit board (PCB) surface hole metallization, plastic electroplating, and microelectronics.
Electroless copper plating on microwave and ceramic hybrid circuit substrates has the advantages of good conductivity, large current load, good thermal conductivity, and good soft soldering performance
Benefits of Copper Plating
Some key copper plating benefits include:
Corrosion Resistance: When a copper layer gets proper application, it keeps the base material from rust and chemical wear.
Improved Conductivity: copper is high conductive., suitable for electronics and electrical applications very conductive.
Enhanced Solderability: Since copper has excellent wettability, it makes soldering very easier during the manufacturing process.
Better Adhesion for Multilayer Coatings: It also acts as a base layer for nickel, gold, & chrome plating, offering quality adhesion and strong bonding.
Problems in Copper Electroplating
Adhesion Problems
Sometimes, the substrate isn’t prepared properly for the electroplating process. In this case, we get to deal with copper adhesion issues.
These issues can lead to flaking or peeling, especially when the substrate is exposed to high-stress environments. The solution lies in cleaning the thing thoroughly for proper activation.
Rough Surface
Electroplated copper coatings may exhibit issues such as copper particles, pits, and whitening. Copper particles in the electroplated layer can result from excessive current, defective fixtures, high sulfuric acid content, or low copper content.
Pits, small holes, or bumps in the copper plating can occur due to contaminants in the plating bath or uneven current density during the electroplating process.
Alkaline Non Cyanide Copper Plating – Future Directions
As environmental concerns grow, non-cyanide copper plating is expected to replace cyanide copper plating and become the dominant method. Common non-cyanide copper plating agents include pyrophosphate, EDTA, citrate, HEDP, amide, ethylenediamine, glycerol, and tartrate.
Despite its advantages, cyanide-free electroplating faces two main challenges: ensuring proper bonding between the substrate and the coating, and maintaining the stability of the plating solution.



