Stainless steel can corrode if free iron or aggressive chemicals compromise the chromium-oxide film. That’s where passivation comes in. This chemical treatment further improves the stainless steel’s corrosion resistance and extends its service life.
Contents
What is Passivation?
Passivation is a chemical surface treatment for stainless steel and other alloys that need corrosion resistance. Passivation removes free iron from the metal surface with nitric or citric acid, allowing a stable, chromium-rich oxide layer to form and improve corrosion resistance.
History of Passivation
The history of passivation dates back to around the 1800s. In 1790, during an electrochemical experiment, Chemist James Keir noticed that iron did not corrode when it was in a bath of strong nitric acid. However, he also noticed that when the same solution was diluted with water, the iron in it corroded. In 1836, a Swiss Chemist by name Christian Friedrich Schönbein concurred with Keir, that if iron was dipped in a strong acid, it could withstand a weak acid.
The popular British electrochemist Michael Faraday came up with a hypothesis in response to Schönbein. He explained that the strong acid formed an oxide skin which might have caused the passive condition. Eventually, chemists and metallurgists explored the idea to further promote passivation.
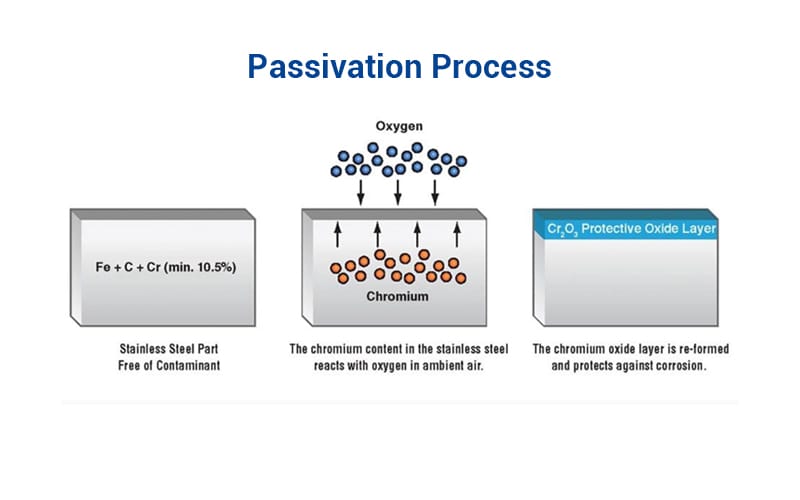
Passivation Process: How It Works
The passivation process usually consists of the following step-by-step breakdown:
1. Cleaning
The first thing to do to ensure a smooth and effective passivation is to clean the material thoroughly by removing oils, greases, dust, and other contaminants using an alkaline cleaner.
2. Chemical Passivation
Here, the component is dipped in the passivation solution, which is either citric or nitric acid. Citric acid solution is usually preferred because of environmental safety issues, though it is slower than nitric acid, except when used with ultrasonic machines. The component is left in the bath for some time and at a particular temperature for the oxide layer to be formed. Stainless steel is typically treated in a nitric acid bath at 35–65 °C for 20–30 minutes.
3. Rinsing and Drying
Once chemical passivation is done, the component is rinsed thoroughly and air dried to get rid of all the acid solution and any other residue.
Types of Passivation
The two main types of chemical passivation are as follows:
1. Citric Acid Passivation
This is an organically produced acid as it is obtained from citrus fruits, which makes it safe for humans to handle and non-toxic for the environment. Citric acid passivation is very effective with almost all steel grades, and it aligns with all the industry standards.
2. Nitric Acid Passivation
Nitric acid is mostly effective for reducing the risk of etching, known as flash attack, during the passivation of stainless steel grades that are less resistant to corrosion. To do this, some chemicals are added, for example, sodium dichromate, while also increasing the concentration and temperature of the nitric acid. However, this makes nitric acid very unsafe for the atmosphere and workers, because the chemical, sodium dichromate, is known to be a carcinogen.
Passivation of Stainless Steel
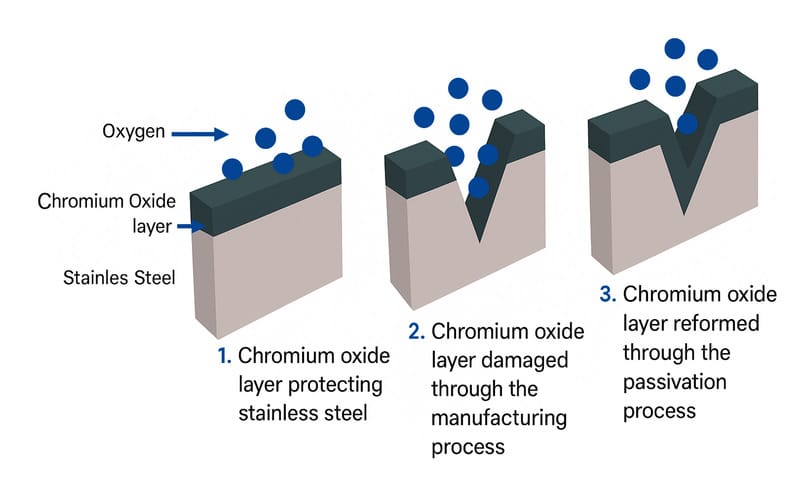
Stainless steel is an alloy of iron, nickel, and chromium; the chromium in it is what gives it the ability to self-passivate. It binds with oxygen to form a passive film or layer, which is naturally resistant to corrosion.
However, it is important to passivate stainless steel when used in processes like machining, welding, or fabrication. This is because some contaminants are introduced in the process, therefore, passivation will need to be done to further strengthen its corrosion resistance. To passivate stainless steel means to treat it with an acid solution that removes free iron and strengthens the protective oxide layer on the surface. Passivation helps it resist pitting and discoloration, which makes it suitable for high-precision applications.
Passivation vs. Pickling
| Feature | Passivation | Pickling |
|---|---|---|
| Purpose | Enhance oxide layer | Remove scale/oxides |
| Acid Strength | Mild (citric/nitric) | Strong (hydrofluoric, nitric) |
| Surface Impact | No etching | Surface is etched |
| Common Use | Post-cleaning | Post-welding |
Benefits of Passivation
- It improves resistance to corrosion and prevents the risk of failures caused by corrosion of metal components when used in certain applications.
- Ensures a longer life span for components.
- Improves the cleanliness of components, thereby making them suitable for medical, food, and other sensitive uses.
- The process is cost-effective.
- Ensures the safety of products and hygiene thanks to its compliance with industry standards, thereby improving quality assurance.
Limitations and Considerations
- Not suitable for all metals (e.g., carbon steel, aluminum).
- Requires proper pre-cleaning to be effective.
- Surface contamination can hinder passive layer formation.
- Re-passivation may be needed after repeated exposure to harsh chemicals or re-machining.
Applications of Passivation
- It plays a vital role in ensuring quality assurance in the pharmaceutical industry.
- Used for food processing and storage.
- Important for the production of components or parts in aerospace applications where corrosion resistance is critical.
- In the medical sector, to make implants and other medical instruments.
- Protects pipelines and valves in the oil and gas industry.
FAQ
1. How Often Should Passivation Be Done?
It depends on usage. Generally it is advised that re-passivation be done yearly, however, components exposed to extreme environments and use should be re-passivated more often.
2. Is Passivation Better Than Coating?
Both processes play important roles; neither one is better than the other. Passivation preserves the natural metal surface, while coatings add a layer. The best choice depends on the application, cost, and desired durability.
3. What Is The Difference Between Pickling and Passivation?
Pickling uses stronger acids to remove oxides and scale by etching the metal.
Passivation focuses on chemically treating the surface to enhance the protective oxide layer without changing the metal’s dimensions.
4. What Are the Industry Standards That Guide Passivation?
ASTM A967: Widely used in the U.S. for stainless steel passivation. Specifies test methods and accepted acid solutions.
ASTM A380: Standard of practice for cleaning, stainless steel passivation, and equipment parts.
BS EN 2516: A British standard for passivating steels and decontaminating alloys with nickel base.
ISO 16048: International standard providing similar guidelines for chemical passivation.
5. Can Passivation Be Removed?
Yes, it can be removed in two ways: The first way is the possibility of the passive layer deteriorating on its own. The second way is to remove it by polishing or welding.



