Is Zinc Magnetic? No, Zinc is not magnetic. However, before you get to know why it isn’t magnetic, let’s introduce the metal briefly. Zinc is one of the elements on the periodic table with the symbol Zn, and it is bluish-white in appearance. To better understand its magnetic properties, we will talk about the way the electrons are arranged in its orbitals. As we progress, you will also see how important zinc is when used in industries like electronics, aerospace, and engineering.
Contents
What Does It Mean for A Material to Be Magnetic?
A material is said to be magnetic if it is attracted to magnets and can become a magnet itself. The magnetism of materials depends on the arrangement of electrons and their movement in the atom. The five classes of magnetic materials are ferromagnetic, ferrimagnetic, antiferromagnetic, paramagnetic, and diamagnetic materials.
The first two classes exhibit strong magnetism e.g. iron. While the last three have very weak magnetism and are non-magnetic e.g. zinc, where zinc falls under diamagnetism. Diamagnetic materials are repelled by a magnetic field because they have filled orbitals with no unpaired electrons. This is why they create a negative magnetization when exposed to an external magnetic field.
Atomic and Electronic Structure of Zinc
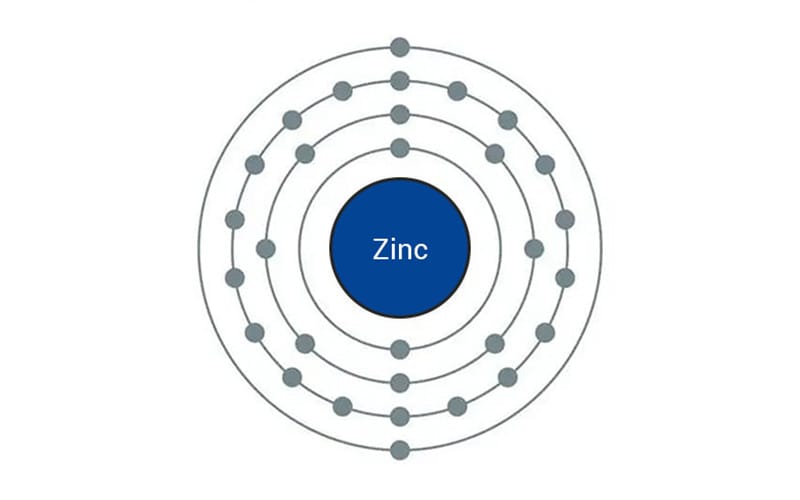
Zinc has an atomic number of 30 which means that it has an electronic configuration of (Ar)3d104s2. This means the last two subshells are full with no electrons left to be paired that can contribute to magnetic moments. When an external magnetic field is applied, it induces a magnetic field which opposes the applied one resulting in a weak repulsive force. Zinc has a very low magnetic susceptibility of about -6 × 10⁻⁵ cm³/mol which means it has a very weak diamagnetism.
Is Zinc Alloy Magnetic?
As earlier mentioned, zinc is not magnetic because of its diamagnetic properties. Similarly, when combined with other materials like copper to form brass, or aluminum, etc. the alloy remains non-magnetic. Except in some cases where the material zinc is alloyed with, has magnetic properties, for example steel. Steel coated with zinc (galvanized) is an example. Here, the steel which is an alloy of iron, is highly attracted to magnets, despite being coated with a thin layer of zinc. In summary, the magnetic properties of zinc alloys depend on the material with which zinc is combined.
Applications and Implications of Zinc as A Non-Magnetic Metal
The fact that zinc is non-magnetic has several practical implications:
Electronics and Electrical Components
Zinc does not interfere with magnetic-sensitive components and is often used in electronic applications where magnetic neutrality is important. Additionally, it is the go-to metal used in fuses and batteries where magnetic neutrality is needed.
Galvanization
Commonly used to coat iron and steel for rust prevention. It doesn’t interfere with the magnetic properties of the coated materials due to its non-magnetic nature. This ability can be crucial in construction and manufacturing.
Aerospace
Due to its lightweight, corrosion-resistance, and strength, it is a desired metal in the manufacturing of aerospace components like brackets, fasteners, etc. It also helps in reducing fuel consumption when used for components that help reduce the weight of the aircraft.
Medical Equipment
Zinc is used to manufacture certain biomedical devices that do not need magnetic properties. An example is the MRI machine, where parts are made with non-magnetic metals to prevent magnetic fields from interfering with the imaging process.
FAQ
1. Does Temperature Affect the Magnetic Behaviour of Zinc?
No, the magnetic behaviour of zinc is not significantly affected by temperature changes. This is because the diamagnetic properties of zinc are inbuilt in its electron structure. Compared to ferromagnetic materials, temperature doesn’t really affect diamagnetism.
2. Can Impurities Influence the Magnetic Properties of Zinc?
Yes, the presence of some impurities like antimony, arsenic, and germanium can create unpaired electrons which will participate in the atomic moments. This usually results in some isolated paramagnetic areas within the material itself.
3. Is There a Difference Between Zinc’s Diamagnetism and Paramagnetism if They Both Are Non-magnetic?
Yes, there is a difference. Although both types of materials react to externally applied magnetic fields, they do so in opposite directions. Zinc as a diamagnetic material is weakly repelled by magnetic fields due to the absence of unpaired electrons. On the other hand, paramagnetic materials are weakly attracted due to the presence of unpaired electrons. The main difference is in their electron configuration.
4. Can Zinc-Coated Materials Be Used for Sensitive Applications Where Magnetism Is Not Needed?
No, even though zinc itself is not magnetic, the underlying metal, for example, steel or iron, is magnetic. Although zinc coating is non-magnetic, galvanized steel remains magnetic due to its iron core. In sensitive applications like MRI where magnetism is undesirable, this would interfere with the images being scanned by causing artefacts.



