Solid state sintering is a heat treatment process in which loose powder is converted into solid coherent components. The process is conducted at elevated temperatures, typically between 70% and 90% of the material’s melting point. This heating leads to the formation of chemical bonds between the particles in the solid state. As this bonding progresses, connecting necks between the particles are formed, the porosity decreases, and the overall density of the material increases.
Contents
How Solid-State Sintering Works?
Neck Formation and Initial Bonding
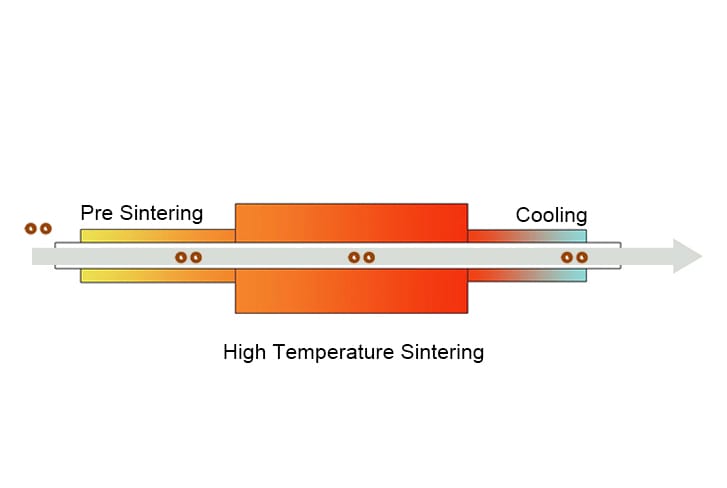
At the initial stage, as soon as sintering begins, the powder particles come into contact with each other, and necks form at their contact points. This neck formation happens through the atomic transport and surface diffusion. At this stage, the coordination number of the particles increases, and neck growth continues until the relative density of 75% is achieved. This stage basically sets the foundation for the densification by maximizing the particle contact.
Diffusion-Controlled Grain Interaction
In second stage, the densification continues through a material transport mechanism, which could be:
- Lattice Diffusion
- Grain Boundary Diffusion
At this stage, the interconnected channels are formed by pores along grain boundaries and around the neck region. These channels shrink, causing an increase in relative density to about 93%. Grain boundary energy begins to play a major role here as it drives grain growth as the system seeks to minimize total interfacial energy. At the end of this stage, the microstructure evolves into a network of polyhedral grains with pores located mainly at three-grain edges.
Densification and Pore Elimination
In the third stage, the remaining pores begin to shrink and break apart into tiny, isolated pockets. As heating continues, the grain develops further, minimizing pores and resulting in a highly dense component. If there are no gases entrapped between the particles the material can reach almost full density. At the end of this stage, the components obtained have remarkable mechanical properties and become strong, highly dense, and close to their final shape.
Applications of Solid-State Sintering
Powder Metal Components
Solid-state sintering has found its application in the manufacturing of powder metal components that require high density and strength. These components include bearings, gears, high-strength structural parts, and more. The process enables complex geometries with good wear resistance and dimensional accuracy.

Ceramics
Solid-state sintering plays a vital role in manufacturing dense and high-performance components using ceramics without relying on a liquid phase. Two major categories of advanced ceramic products can be produced using solid-state sintering, including:
- Electromagnetic ceramics: These include applications in electric, magnetic, and optical fields, such as capacitors, insulators, and laser materials.
- Structural ceramics: For thermomechanical uses like cutting tools, biomedical implants, and engine components.
The process ensures improved strength, thermal resistance, and reliability across both categories.
Refractory Materials
Solid state sintering also helps in producing a number of refractory materials components which can endure extreme heat and mechanical stress. Refractory materials that are processed through solid-state sintering include tungsten (W), molybdenum (Mo), tantalum (Ta), niobium (Nb), and zirconia (ZrO₂), as well as alumina (Al₂O₃). The refractory components produced through this technique are widely used in aerospace turbine parts, crucibles, heating elements, and radiation shields.
Benefits of Using Solid-State Sintering
Preservation of Material Integrity
In solid-state sintering, material chemical and structural integrity is preserved as the powder material consolidates without melting, typically in an inert atmosphere. This approach prevents drastic thermal gradients, phase changes, oxidation, contamination, and distortions. As a result, it maintains dimensional accuracy and protects the material from structural damage.
Enhanced Mechanical Properties
Solid state sintering enhances the integrity, strength, and durability of the material as it causes an increase in density of the material by the formation of sintered necks between the particles. Not only does this solid-state sintering reduce surface oxides and remove residual lubricants, but it also produces a denser and more uniform microstructure.
Controlled Microstructure
Another key advantage of solid-state sintering is its ability to produce a finely controlled microstructure. Manufacturers can achieve a dense structure with uniformly distributed grains by carefully adjusting factors such as particle size, sintering temperature, and holding time.
For example, studies on oxide-dispersion-strengthened (ODS) tungsten alloys have shown that controlling the microstructure prevents excessive grain growth while maintaining the high density.
Additionally, well-regulated microstructure also improves fracture resistance and overall durability, making sintered components ideal for critical applications in aerospace, defense, and advanced engineering.
Cost Advantages
Lower Energy Consumption
Last but not least, solid-state sintering is a cost-efficient process, as it requires significantly less energy by operating below the material’s melting temperature.
Minimal Material Wastage and Secondary Operation
Additionally, the process generates minimal material wastage and typically reduces the need for extensive secondary operations, lowering overall manufacturing costs.
Limitations of Solid-State Sintering
Long Sintering Time
Solid-state sintering often requires prolonged processing durations, especially for materials with high melting points or low atomic diffusivity. This is because of the slow rate of atomic migration required for particle bonding and densification.
Incomplete Densification
Since solid state sintering does not occur in the presence of any liquid that is why it’s challenging to achieve full densification of the material. This affects the mechanical integrity and performance of sintered material. For example, in the sintering of SiC ceramics, solid-state sintering primarily relies on boron-carbon-based additives to facilitate densification. At the same time, achieving full densification solely through solid-state mechanisms can result in residual porosity and reduced mechanical properties.
High Temperature
To facilitate atomic diffusion, solid-state sintering necessitates high temperatures. This can lead to issues such as grain growth or distortion. These thermal effects may introduce defects and reduce the overall performance of the sintered part.
Solid State Sintering Vs Liquid Phase Sintering
The table below compares solid state sintering and liquid phase sintering.
| Attribute | Solid-State Sintering | Liquid-State Sintering |
|---|---|---|
| Definition | Densification occurs entirely in the solid phase below melting point. | A liquid phase forms during sintering to promote densification. |
| Temperature | 0.7–0.9 Tm (no melting). | 0.8–0.98 Tm (partial melting). |
| Mechanism | Atomic diffusion—surface, grain boundary, and volume diffusion. | Liquid wets solids, enabling rearrangement and solution–precipitation. |
| Driving Force | Reduction of solid surface energy. | Capillary forces and liquid wetting. |
| Densification Rate | Slow; controlled by diffusion. | Fast; assisted by liquid flow. |
| Microstructure | Fine, uniform grains; high dimensional stability. | Coarser grains; possible segregation or distortion. |
| Materials | Fe, Cu, W, Mo, stainless steel, Al₂O₃, Si₃N₄. | WC–Co, Cu–Sn, cermets, infiltrated bronzes. |
| Advantages | Stable shape, clean microstructure, strong bonding. | High density, lower temperature, good phase bonding. |
| Limitations | Incomplete densification possible. | Risk of distortion or uneven liquid distribution. |
| Applications | Structural PM parts, gears, bushings, filters. | Hardmetals, cutting tools, bronze bearings. |



