The theoretical density of graphite in 2.26 g/cm³, and in 2260 kg/m³.
The density of graphite affects its mechanical strength, thermal conductivity, and electrical properties. Engineers use density as a key indicator to determine whether graphite is suitable for a certain application.
For example, high-density graphite can be used as molds, battery materials, and bearings. Low-density graphite is utilized as lubricant for metal tooling, thermal insulation material.
You may find that graphite density is important when selecting graphite materials for use as electrodes, refractory materials, or lubricants.

Contents
How to Measure the Theoretical Density of Graphite
Before you can measure the density, you need to understand the structure of graphite.
Graphite has a layered hexagonal crystal structure. Each layer consists of carbon atoms arranged in a honeycomb lattice, with weak van der Waals forces between the layers.
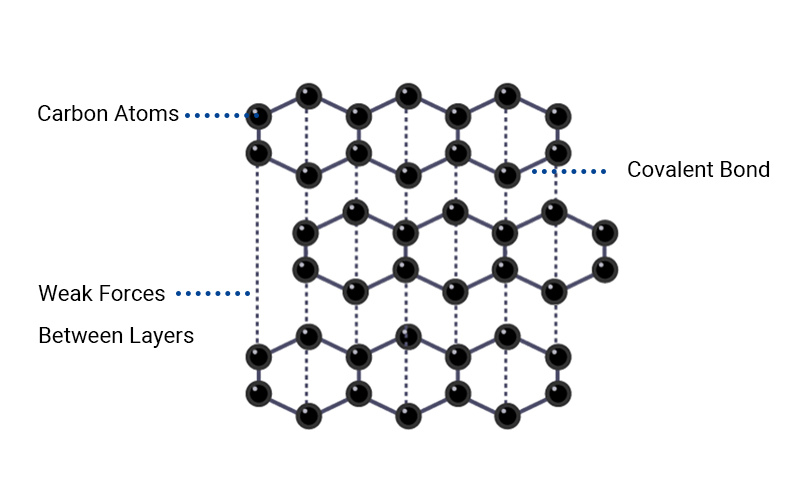
The theoretical density of graphite is close to 2.26 g/cm3, which you can calculate in the following ways:
Direct measurement:
Calculate the density by directly measuring the mass and volume.
You can measure the mass of a graphite sample using a precision pan balance.
Volume: For graphite samples with regular geometric shapes, you can calculate directly. For irregular shapes, you can measure by displacement.
Indirect measurement:
Gas specific gravity method
Measure the volume of a graphite sample by gas displacement (e.g. helium).
The specific steps are as follows:
- Place the graphite sample in a chamber of known volume.
- Pass an inert gas (helium) and measure the pressure change.
- Use Boyle’s law to calculate the volume of the sample.
- Combine with mass) to find density
You can also use XRD to determine the lattice parameters of graphite and then calculate the theoretical density.
Density of Different Graphite Products
Common types of graphite are as follows.
Natural Flake Graphite
You may find flake graphite in metamorphic rocks, they exist in lamella or scaly form limestone, gneisses, and schists.
Synthetic Graphite
Synthetic graphite, also known as artificial graphite, is usually made from coal tar pitch or petroleum by heat treatment. Synthetic graphite usually has higher resistivity and porosity, and lower density between about 1.6 and 1.9 g/cm3.
Isostatically Press Graphite
Isostatically graphite has a density of about 1.7 to 1.9, and is a fine grain graphite with fine aggregate. It has high electrical conductivity, high thermal conductivity, and extremely high heat resistance, and is used in photovoltaics, semiconductors, casting, etc.
Extruded Graphite
Extruded graphite generally has a density of 1.55 to 1.72 g/cm3, with a coarser grain size and a lower strength. But it has high thermal conductivity and electrical conductivity and is often used in solution processing, casting molds, etc.
The table below shows the density of different types of graphite products.
| Types of Graphite | Density (g/cm³) |
|---|---|
| Natural Graphite | 2.2 |
| Synthetic Graphite | 1.6 to 1.9 |
| Isostatic Graphite | 1.7 to 1.9 |
| Extruded Graphite | 1.55 to 1.72 |
| Graphite Powder (Fine Grain) | 1.83 |
| Graphite Powder (Medium Grain) | 1.72 to 1.90 |
| Graphite Powder (Coarse Grain) | 1.5 to 1.8 |
| Graphite Sheet | 0.85 |
| Expanded Graphite | 2.2 |
Main Factors Affecting Graphite Density
The density of graphite, a crystalline form of carbon, is influenced by factors related to its atomic structure, composition, and external conditions.
Following are some of the main factors that affect graphite density.
Interlayer Spacing
Graphite consists of stacked hexagonal carbon layers held by weak van der Waals forces. Smaller interlayer spacing reduces volume, increasing density (like compacted graphite). Larger spacing (e.g., in expanded graphite) decreases density.
Crystallinity
Highly crystalline graphite (ordered structure) has fewer defects and voids, leading to higher density. Amorphous graphite (disordered) has lower density due to irregular packing.
Purity
Some impurities, like diamond or amorphous carbon) alter density. Diamond impurities increase density, while amorphous carbon lowers it.
Insertion of molecules or ions between layers adds mass, potentially increasing density.
Particle Size and Shape
Graphite powders with a uniform particle size distribution pack more efficiently than irregular or widely varying particles, resulting in a higher overall density.
Manufacturing Process
The density of compacted graphite increases. The high temperatures of the sintering process also reduce the porosity of graphite.
High-temperature annealing may improve crystallinity, increasing density, which milling may introduce defects, lowering density.
Comparing the Density of Diamond and Graphite
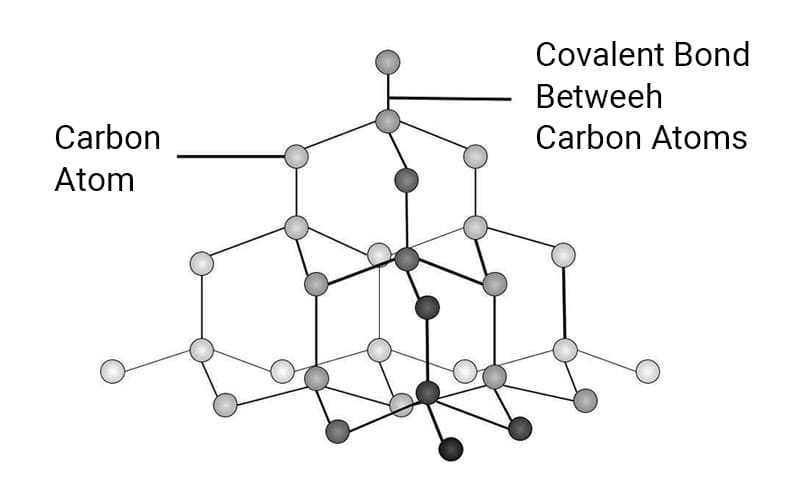
The crystal structure of diamond is tetrahedral (3D network).
Each carbon atom forms four strong covalent bonds with neighboring atoms, forming a rigid and tightly packed lattice. The 3D tetrahedral structure of diamond allows atoms to occupy space efficiently, minimizing voids.
While the crystal structure of graphite is hexagonal layered (2D sheet).
Each carbon atom forms three strong covalent bonds on a plane (forming a hexagonal ring), and the interlayer van der Waals forces are weak. Due to the weak interlayer forces, the layers of graphite are farther apart, so the volume is increased for the same mass.
Density differences lead to variations in the physical properties of graphite and diamond.
Graphite’s low density and layered structure make it lubricious and conductive, and it is incorporated into pencils, lubricants, and battery electrodes. Diamond’s high density makes it hard and conducts heat well. This makes it ideal for cutting tools or abrasives.
FAQ
1. What is High Density Graphite?
High-density graphite is a material with high strength and fine microstructure, with a bulk density of 1.8 g/cm³.
Due to its ability to withstand extremely high temperatures, high strength, thermal conductivity and electrical conductivity, high-density graphite has a wide range of applications, such as lithium-ion battery anodes, metal casting molds, heat exchangers, bearings and bushings in high-temperature or friction applications.



